Hox Is a Gene Family That Determines Skin or Cuticle Coloration in a Wide Range of Animals.
Introduction
Homeobox genes encode transcription factors that demark DNA in a sequence-specific fashion through the homeodomain motif and control the expression of their target genes in a huge range of developmental processes (Duboule, 1994). It is difficult to find a developmental cistron network in animals that does non include a homeobox gene. These genes are taxonomically widespread, being plant in animals, plants, fungi, and protists (Derelle et al., 2007; Mukherjee et al., 2009; de Mendoza et al., 2013; Mishra and Saran, 2015) and are thought to take evolved from some sort of Helix-turn-Helix protein similar to those establish in prokaryotes (Laughon and Scott, 1984; Kenchappa et al., 2013). Focusing on the homeobox genes of animals, eleven classes of gene families are commonly recognized: ANTP, PRD, LIM, POU, HNF, SINE, TALE, CUT, PROS, ZF, and CERS (Holland et al., 2007). Several of these classes are distinct to animals and by implication are likely to be linked to the development of aspects of animal-specific biology (meet Figure ane; Larroux et al., 2008; Degnan et al., 2009; Suga et al., 2013) [but of form, not all animal-specific biology is entirely owing to homeobox genes and other animal-specific genes exist (King et al., 2008; Suga et al., 2013)].
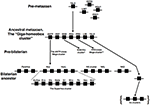
Figure i. Evolution of the hypothetical metazoan "Giga-homeobox cluster." Prior to the origin of animals there were only a small number of homeobox genes, including TALE and CERS-class genes along with several further genes of uncertain affinities (Sebé-Pedrós et al., 2011). The genomic arrangement of these genes is also unclear. In an ancestral metazoan a amassed array of homeobox genes likely existed, consisting of the precursors of several classes: the "Giga-homeobox cluster." The deduction of the composition of this array is described in the text. Further members of this assortment may be revealed by analyses of additional metazoan genome sequences. Sub-components of this Giga-cluster include the ANTP-class Mega-cluster (run into text for details; Pollard and Kingdom of the netherlands, 2000), which had dispersed into at least iv sub-components distributed on distinct chromosomes by the time of the bilaterian (protostome–deuterostome) ancestor (Hui et al., 2012). One of these sub-components was the SuperHox cluster, composed of the true Hox genes (EuHox genes) and several additional ANTP-class genes that were Hox-linked (HoxL). A second sub-component was the "NK cluster" genes with several NK-linked (NKL) genes (see text for details). Farther gene clusters deriving from within the Giga-cluster included the SINE/Half-dozen cluster and the PRD-class Mega-cluster (see text for details). The Iroquois/Irx cluster is different from the other clusters described hither because it expanded to a three-gene cluster independently in several distinct lineages (denoted past the brackets), most likely from a unmarried gene state in the bilaterian antecedent (see text for details). Continuous horizontal lines indicate clustering on the same chromosome. The single asterisk denotes that further details are provided in the text and in Figure 2. The double asterisk denotes that further details are provided in the text and Figure 3.
Another notable feature of animal homeobox genes is that a number of them exist in clusters that are widespread across the animal kingdom. These include clusters of genes from the ANTP-course (e.g., Hox, ParaHox, NK, Mega-homeobox, and SuperHox clusters), the PRD-class (the HRO cluster and its extension), the TALE-class (Irx cluster), and the SINE-class (SIX cluster), as well equally an intriguing "pharyngeal"gene cluster composed of dissimilar classes of homeobox gene as well as other gene families (Garcia-Fernàndez, 2005; Butts et al., 2008; Mazza et al., 2010; Gómez-Marín et al., 2015; Simakov et al., 2015; and see below). The limerick of these clusters and their retention in some beast lineages, just not others, has been the focus of much involvement every bit a possible route to insights into the evolution of animate being development as well every bit genome arrangement and architecture. Here I provide an overview of animal homeobox cistron clusters and the hypotheses linked to their development. I focus on gene clusters with deep evolutionary history in the animals that have been conserved across multiple phyla ("main clustering"), and contrast these with genes existence rearranged to form a cluster that was not nowadays ancestrally ("secondary clustering"). I volition avoid give-and-take of lineage-specific instances of factor duplication that have produced, for example, neighboring paralogs of a particular homeobox family (east.g., mammalian examples summarized in Holland, 2013), except for the distinctive case of the Irx cistron clusters (see below). Since the evolution of the organization of the Hox cluster has been extensively written about elsewhere (east.g., Monteiro and Ferrier, 2006; Duboule, 2007; Ferrier, 2010, 2012; Ikuta, 2011) and to a lesser extent its evolutionary sister the ParaHox cluster (due east.g., Ferrier and Holland, 2001; Ferrier, in printing), I volition focus on other homeobox clusters hither.
The ANTP-course Mega-homeobox Cluster within a Homeobox Superclass Giga-cluster
The Mega-homeobox cluster was kickoff hypothesized by Pollard and The netherlands (2000) on the footing of an analysis of the and so newly available human genome sequence (reviewed in Garcia-Fernàndez, 2005). This hypothesized ancestral cluster of ANTP-form genes includes the well-known Hox genes, too as the ParaHox genes forth with many other ANTP-class families (Pollard and Holland, 2000; Garcia-Fernàndez, 2005). The hypothesis involves the ANTP-grade genes evolving via a series of tandem duplications that generated all of the precursors to each of the ANTP-class families, such that there is a clustered assortment of these family precursor genes together in a Mega-cluster at some point early in brute development. Following the origin of this Mega-cluster it is supposed that it started to break apart during evolution, to leave the sub-components now observed in genomes like that of amphioxus (Castro and Kingdom of the netherlands, 2003), with the Hox cluster and several associated families on one chromosome, the ParaHox cluster on some other chromosome and the NK cluster genes on a tertiary chromosome (Pollard and Holland, 2000; Castro and Holland, 2003; Hui et al., 2012).
The Mega-cluster hypothesis was mainly built on the ascertainment that Dlx genes and Msx4 are linked to Hox genes in mammals (Pollard and Holland, 2000). This was thought to be significant considering these genes supposedly had greater sequence similarity to the NK cluster genes (see below) than to the Hox genes, which was taken equally indicative of an ancestral linkage of all of the Hox and NK cluster genes. The Msx4 data was subsequently excluded when it was establish that this factor probably resulted from a retrotransposition event (Castro and Holland, 2003), so that its genomic location in vertebrates cannot necessarily be taken equally indicative of the ancestral pre-vertebrate location. This is because such an origin via retrotransposition was singled-out from the origins of the other vertebrate Msx paralogs during the 2 rounds of whole genome duplication events (the so-chosen 2R events) that occurred at the origin of the vertebrates. Thus, the locations of the other Msx paralogs, rather than Msx4, are more likely to be indicative of an bequeathed Msx genomic location. Msx1 and Msx2 (and Msx3 in mouse) are linked to genes of the NK cluster (Pollard and Holland, 2000), which is discussed further below.
The suitability of Dlx every bit the foundation for the Mega-cluster hypothesis has now also been questioned (Hui et al., 2012). The part of Dlx in the hypothesis hinged on the view that its sequence was closer to those of the NK gene families (placing it within the NK subclass), which led to Dlx sometimes being referred to as an NK-like (NKL) factor (reviewed in Ferrier, 2008). With further taxonomic sampling and a greater diversity of homeobox genes being incorporated into molecular phylogenies and classification analyses, information technology became articulate that the NKL categorization of Dlx was not justified (Ferrier, 2008; Hui et al., 2012). Since the molecular phylogenies of the ANTP-course homeobox genes no longer provided articulate support for the Mega-cluster hypothesis, Hui et al. (2012) attempted a different approach, of just determining the genomic linkage patterns of ANTP-class genes with the aim of determining which are Hox-linked (HoxL) and which are NK-linked (NKL). This alter in definition of HoxL and NKL to reverberate unambiguous linkage of genes, rather than poorly resolved or unstable phylogenetic relationships of homeobox families, was the precursor to assessing whether singled-out animal lineages (such as the deuterostome amphioxus and the protostome Platynereis dumerilii) had distinct remains of the hypothetical Mega-cluster that represented the cluster breaking in different places in independent lineages. If ii distinct, but overlapping, patterns of linkage had been found in these ii animals then support for the Mega-cluster hypothesis would take been obtained. However, Hui et al. (2012) instead fabricated the surprising discovery that the distribution of the ANTP-class genes across the chromosomes of P. dumerilii is largely identical to the distribution in amphioxus. This may have intriguing implications for potential functional reasons for the retained clustering of some of these homeobox genes across such large evolutionary distances, such as the subsets of NK genes (discussed in Hui et al., 2012). Nevertheless, support for the Mega-cluster hypothesis was non obtained. Instead, it appears that the Mega-cluster had either already cleaved apart into the distinct linkage groups and patterns that are at present present in both P. dumerilii and amphioxus past the time of their concluding common ancestor (the protostome–deuterostome ancestor), or the Mega-cluster never existed in the kickoff place. Perhaps the various ANTP-course families that are considered within the context of the Mega-cluster hypothesis started to disperse across an bequeathed (pre-bilaterian) genome before all of these families had come into beingness, such that instead of a single Mega-cluster at that place were several sub-clusters.
Additional members of the Mega-cluster or "Mega sub-clusters" are at present being establish every bit farther whole genome sequences become available. These tight linkages and clustering are also now extending beyond the ANTP-class. For example, the sine oculis (So) cistron from the SINE-course clusters with the ANTP-grade genes Empty spiracles (Ems) and Intermediate neuroblasts defective-b (Ind-b) in the myriapod Strigamia maritima, as well as the Hmbox gene (from the HNF-class) clustering with the ANTP-class genes Exex, Nedx, and Buttonless-a (Btn-a) (Chipman et al., 2014). The first of these ii Due south. maritima examples may in plough relate to the SINE/Half-dozen gene clusters (see beneath), whilst the 2d case constitutes an extension of a particular sub-component of the Mega-cluster (or one of the "Mega sub-clusters"), the SuperHox cluster (see below). For further discussion of the Southward. maritima homeobox linkages, see the supplementary text in Chipman et al. (2014).
At that place are additional examples found in not-bilaterian lineages, such as clustering of a POU-class and ANTP-form gene in a cnidarian (Kamm and Schierwater, 2007). Besides, a number of intriguing instances of homeobox clustering involving unlike gene classes are plant in the placozoan Trichoplax adhaerens (Schierwater et al., 2008). These include the PRD-class gene Goosecoid (Gsc) being clustered with ANTP-class genes of NK families, the HNF-class factor (Hnf) existence clustered with a PRD-course cistron (Prd/Pax-like), there is a cluster of ii PRD-class genes (Arx1 and Arx2) with a TALE-course gene (Pknox) and there are ii instances of a LIM-class gene being clustered with a TALE-course gene (Lim2/9 with Pbx/PBC, and Lim1/5 with Meis). In that location is also an example of a LIM-class cluster that, thus far, seems distinctive for T. adhaerens (Srivastava et al., 2010). These sorts of intriguing single cases of homeobox gene clustering clearly demand to be examined more widely, to investigate whether they occur in multiple species. This and then volition decide how they chronicle to evolution of master or secondary clustering, discussed further below. In this vein, at that place are also a couple of instances of PRD-class gene clustering in T. adhaerens that, in dissimilarity to the LIM cluster, do relate to more taxonomically-widespread clusters (see below).
Since several of these different homeobox cistron classes are specific to the animals, it is reasonable to presume that they arose via duplications (probably tandem) from an ancestral metazoan homeobox cistron. This likely resulted in an extensive array of unlike homeobox genes in an early fauna ancestor, containing representatives of the precursors for most (perhaps all) of the animate being homeobox classes. Some of these genes remained amassed and some of these conserved clusters were retained into modern-day lineages due to functional constraints. These constraints probably included long-range regulatory mechanisms acting across multiple genes, either directly on multiple promoters as occurs in Hox gene regulation (e.g., Tarchini and Duboule, 2006) or indirectly with long-range enhancers spanning bystander genes (Kikuta et al., 2007). Further study of the diversity of homeobox gene clusters across a diversity of animal lineages is thus likely to pb to new insights into the command mechanisms of clustered cistron regulation. Furthermore, we can now go beyond the ANTP-grade Mega-cluster hypothesis to a homeobox superclass "Giga-cluster" hypothesis (Figure 1).
The SuperHox Cluster
The SuperHox cluster was beginning described by Butts et al. (2008). This cluster was composed of 8 ANTP-class genes that could be deduced equally existence neighbors of the Hox factor cluster in the bilaterian antecedent, including Mox, Hex, Ro, Mnx, En, Nedx, Dlx, and Evx alongside Hox. The SuperHox cluster was thus seen as a specific sub-component of the hypothetical Mega-cluster and, as with the Mega-cluster, the SuperHox has since been breaking apart during evolution in dissimilar places on distinct creature lineages. The 15-gene SuperHox cluster, which contained the eight genes listed to a higher place alongside seven truthful Hox genes (or "EuHox" genes) in the bilaterian ancestor (Balavoine et al., 2002), was deduced from comparisons of the conservatively evolving genomes of amphioxus and the carmine flour beetle (Tribolium castaneum; Butts et al., 2008). An important assumption underpinned the construction of this cluster from the amphioxus and beetle information; since these genes all belong to the ANTP-course and hence have evolved from each other via duplication, then it is nearly likely that these duplications were tandem and that the ancestral genes for each family first arose as close genomic neighbors. Thus, ANTP-class genes that are found as shut neighbors in extant animals, similar amphioxus and the cerise flour protrude, are more probable to reflect descent from a state in which the genes were neighbors, rather than these genes first evolving as shut neighbors, and so dispersing around the genome and finally coming back together to be close neighbors secondarily ("close" being taken as <fourscore kb in the case of the SuperHox deductions; Butts et al., 2008). Whether this assumption is justified volition be returned to below, when discussing the NK and pharyngeal clusters.
A further sub-component of the hypothetical Mega-cluster in its initial formulation was the EHGbox cluster, composed of En, HB9, and Gbx (Pollard and Holland, 2000). Given the appealing sounding acronym for this gene cluster information technology is perhaps unfortunate that the HB9 genes have since been renamed to Motorneuron homeobox (Mnx) (Ferrier et al., 2001). Perhaps in view of this the cluster should also be renamed, to the GEMbox cluster. However, it could as well be argued that the idea of an EHGbox/GEMbox cluster tin can be dispensed with anyway. This is considering the molecular distances between the genes are in the order of Megabases in mammals, and hence are much larger than the kilobase distances that found the close neighbour relationships since used for deduction of the SuperHox cluster, for instance. Also, the En and Mnx genes of the EHGbox/GEMbox have been subsumed within the SuperHox cluster (Butts et al., 2008).
Further genome sequencing projects have enabled the composition of the SuperHox cluster to be extended slightly. The inclusion of a non-ANTP-class gene, Hmbox (from the HNF-form), has already been mentioned above, in the context of the Mega-cluster and contempo data from the myriapod Due south. maritima (Chipman et al., 2014).
SINE/Six Gene Clusters: CTCF-mediated TADs
If we move out of the ANTP-grade nosotros find farther examples of homeobox factor clusters. One such cluster is that of the SINE-form genes from the Six1/ii, Six4/5, and Six3/6 families. This cluster again is likely to have an ancient beginnings in animal evolution. Six3/6 is clustered with Six1/2 in the non-bilaterian T. adhaerens (which lacks a Six4/five factor; Schierwater et al., 2008). The total cluster of three genes is found across several bilaterians, including the hemichordates (Simakov et al., 2015), lophotrochozoans (Irimia et al., 2012; Simakov et al., 2013), an echinoderm, and vertebrates (Gómez-Marín et al., 2015), whilst the cluster has dispersed in insects (Effigy 2). The state of affairs in vertebrates has been made more complex by the whole genome duplications that occurred at the origin of vertebrates, followed by a further duplication early on in teleost evolution. Some cistron loss followed each of these whole genome duplications such that in tetrapods there tends to be two SINE clusters, one of Six1, Six6, and Six4 and a 2d of Six3 and Six2, with a third locus containing just a single SIX factor, Six5 (Figure two). In a teleost like the zebrafish there are five clusters, 2 of which contain 3 genes whilst three clusters possess merely two genes (forth with a farther locus containing i lone SIX gene; Effigy two).
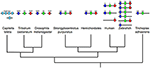
Figure 2. Evolution of the SINE/Six cluster. A cluster of at least two genes existed in not-bilaterians (eastward.g., T. adhaerens). Gene clusters are widespread across the bilaterians, including deuterostomes (e.m., S. purpuratus, hemichordates, humans, and zebrafish) and at to the lowest degree one lophotrochozoan (the annelid, C. teleta), but non in the insects (e.g., D. melanogaster and T. castaneum) in which the genes are dispersed across separate chromosomes. Closer report is required to resolve the precise orthologs of the C. teleta genes relative to specific gene families (denoted by the pale blue coloration). Nighttime bluish coloration denotes the optix/Six3/half dozen gene family, greenish the So/Six1/ii gene family, and red the Six4/5 gene family unit. All information on gene identification and genomic locations is taken from both Ensembl (http://www.ensembl.org/index.html) and HomeoDB (Zhong et al., 2008; Zhong and Holland, 2011), the Capitella genome portal (http://genome.jgi.doe.gov/Capca1/Capca1.home.html), Gómez-Marín et al. (2015) and Simakov et al. (2015).
Four of the five SINE clusters of zebrafish were recently shown to be bailiwick to long-range regulatory processes that result in Topologically Associated Domains (TADs), the organization of which is like in both mouse and sea urchin (Gómez-Marín et al., 2015). These TADs are bordered by CCCTC-bounden factor (CTCF) sites. This organization, with CTCF-bordered TADs operating over homeobox gene clusters, has also been found for the Hox clusters (Gómez-Díaz and Corces, 2014; Maeda and Karch, 2015; Narendra et al., 2015), and is thus likely to be a rather general machinery at work in such gene clusters.
The TALE-class Iroquois/Irx Cluster: Contained Cluster Expansions
The TALE-class of homeobox genes is one of the few classes that evolved prior to the origin of the animals (Degnan et al., 2009; Suga et al., 2013; see Effigy i). Within the TALE-class, the Iroquois/Irx genes tend to be clustered in animals. This cistron cluster is a little different from the others discussed here. Although, three-factor Irx clusters are widespread beyond the animal kingdom there appear to exist several cases of them having evolved independently, via singled-out instances of tandem duplication that in several cases have produced gene clusters of three genes. Thus, although Irx clusters are widespread they are not entirely homologous across all lineages, in the sense that the clusters have been produced from evolutionarily independent factor duplication events. Comparable processes of lineage-specific tandem gene duplication within homeobox gene clusters can be seen in other clusters, such equally the Hox (Ferrier, 2012). But the distinctive and intriguing difference virtually the Irx clusters is that they accept repeatedly settled on a three-gene composition. This has happened independently for vertebrates, amphioxus, drosophilids, a myriapod, and an annelid (Irimia et al., 2008; Takatori et al., 2008; Kerner et al., 2009; Maeso et al., 2012; Chipman et al., 2014). Why this might be so still remains a mystery.
PRD-class Clusters: Remains of a PRD-class Mega-cluster?
Mazza et al. (2010) identified the HRO cluster of PRD-grade genes in Cnidaria and protostomes, including insects and molluscs. This cluster is equanimous of the genes Homeobrain (Hbn), Rax/Rx, and Orthopedia (Otp). At least part of the cluster is even more than ancient than the cnidarian-bilaterian ancestor as Hbn and Otp are also clustered in the placozoan T. adhaerens (Mazza et al., 2010). Also, elements of the HRO cluster are now known to exist more widespread in protostomes than initially described. For instance, more contempo whole genome sequencing projects similar that of the myriapod S. maritima have revealed that this arthropod has as well retained the HRO cluster (Chipman et al., 2014).
Intriguingly, this HRO cluster exhibits temporal collinearity in the cnidarian Nematostella vectensis (Mazza et al., 2010). That is, the guild of the genes along the chromosome corresponds to the order in which they are activated during development. Temporal collinearity has besides been hypothesized to be the chief underlying reason for the maintenance of intact, ordered Hox and ParaHox clusters (Ferrier and Kingdom of the netherlands, 2002; Ferrier and Minguillón, 2003; Monteiro and Ferrier, 2006). Thus, there is the potential that deeper mechanistic understanding of temporal collinearity can be obtained past comparisons across all iii homeobox clusters: Hox, ParaHox, and HRO.
Clustering of PRD-class genes is not confined to the HRO cluster. The clustering of Goosecoid (Gsc) and Otx was noted in amphioxus (Putnam et al., 2008; Takatori et al., 2008) and the hemichordate genome sequences analyzed recently, reveal that in i species (Ptychodera flava) Gsc also clusters with Otx, but in some other species (Saccoglossus kowalevskii) Gsc instead clusters with Otp, Rx, Hbn, and Islet (Isl) (all of which are PRD-class genes except Isl, which is LIM-grade; Simakov et al., 2015). 2 things are noteworthy hither. First, it volition be important to independently bank check the Saccoglossus gene arrangement, particularly the location of Gsc. 2nd, the gene classification risks causing confusion and in extended Figure 4 of Simakov et al. (2015), the authors take depicted the cluster containing an Arx gene, when in fact the gene should be named Hbn or Arx-like on the basis of its sequence. Arx is a distinct family unit from Hbn/Arx-like, every bit seen in the cnidarian Nematostella vectensis (Ryan et al., 2006; Table one).
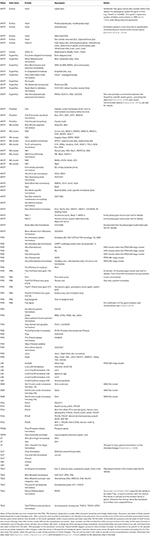
Table 1. Homeobox families present in the protostome–deuterostome antecedent (PDA).
Looking deeper in beast evolution, Schierwater et al. (2008) noted ii instances of PRD-class clustering in T. adhaerens: PaxB with Pitx and Ebx/Arx-similar with Otp (this second cluster also containing the LIM-class cistron Isl). The Ebx/Arx-similar gene of Schierwater et al. (2008) is equivalent to the Hbn factor of Mazza et al. (2010). This then, in combination with the new hemichordate information, establishes the clustering of Otp with both Hbn/Arx-like and Isl as an ancient cluster that has been conserved from earlier the start of the Cambrian, over 541 million years ago. Furthermore, in combination with the data on the HRO PRD-class cluster of cnidarians and selected bilaterians, information technology is possible to deduce an ancestral extended PRD-LIM class cluster including Hbn, Rx, Otp, Gsc, Otx, and Isl (Effigy three). By comparison to the large ancestral array hypothesized for the ANTP-form (see in a higher place), nosotros perchance should now too view the PRD-grade as having evolved via a Mega-cluster array as well (which in turn was as well a sub-component of the Giga-cluster outlined higher up).

Figure 3. Composition of the PRD/LIM-form Mega-cluster. Specific instances of gene clustering are listed against specific taxa, which when considered together permit the deduction of the PRD/LIM-class Mega-cluster. These animals include non-bilaterians (T. adhaerens and Due north. vectensis), protostomes, and deuterostomes (hemichordates and amphioxus). Nigh members of the array are PRD-class genes (blackness boxes), but there is also a single member of the LIM-class (white box). The Pitx and Pax (PaxB) clustering is constitute in T. adhaerens, merely is not reported for some other fauna as withal, hence the question mark to denote the ambiguity equally to whether these PRD-class genes can be included in the PRD/LIM-grade Mega-cluster. The HRO cluster is the PRD-form cluster originally described by Mazza et al. (2010). The figure only shows established instances of clustering arrangements described in the literature (see text for details). Lack of a gene alongside a taxon does not necessarily stand for absence of the gene from the genome of that species, except in the instance of Rax/Rx for T. adhaerens, which was non found in the placozoan genome by Mazza et al. (2010) (denoted by "X").
The NK Cluster: An Ancestral Cluster Breaking Apart or Dispersed Genes Meeting?
If nosotros now render to the ANTP-form, a cluster of NK homeobox genes has been known in insects like Drosophila melanogaster for a number of years, with a prominent role in patterning mesoderm development (Jagla et al., 2001). The limerick of the bequeathed insect NK cluster has been deduced by consideration of a range of species, such that the "NK cluster" genes can be considered to be a selection from Msx/Drop, tin/NK4, bap/NK3, Lbx, Tlx/C15, slou/NK1, and Hmx/NK5, with subsets of this group forming clusters in detail extant species (Luke et al., 2003; Wotton et al., 2009). Combining this insect data with chordate information has led to the hypothesis that the NK cluster in the bilaterian ancestor included all of the insect "NK cluster" genes as well as NK6 and NK7 (Wotton et al., 2009; Holland, 2013). An NK cluster has also been described for the sponge Amphimedon queenslandica (Larroux et al., 2007). More than recently an NK cluster has been identified in hemichordate deuterostomes, with the limerick of Hmx/Nkx5-Msx-Nkx3.two-Nkx4-Lbx-Hex when both Saccoglossus kowalevskii and Ptychodera flava are considered together (meet Supplementary Extended Effigy 4 in Simakov et al., 2015). This is the about all-encompassing deuterostome NK cluster known, and it intriguingly includes the Hex cistron. This gene is also a member of the SuperHox cluster as well equally the Mega- and Giga-clusters (see above), thus possibly helping to tie all of these clusters together.
In many other species, sub-components of the NK cluster are found every bit "fragments" of the canonical cluster defined from the insect–chordate comparisons. The assumption is that an ancestral creature had an intact NK cluster and this cluster largely remained intact on the lineage leading to insects, only on the lophotrochozoan and deuterostome lineages the cluster started to break apart. Intriguingly, these breaks are often in similar places, such that the aforementioned sub-groups of "NK cluster" genes are found as close genomic neighbors across phylogenetically disparate species (Luke et al., 2003; Wotton et al., 2009; Hui et al., 2012). A likely explanation for the retention of certain sub-components of the NK cluster is that multigenic or shared regulatory elements existed in the ancestral cluster which have been retained into extant lineages. This then restricts the locations within the cluster at which viable breaks can exist fabricated. Prove for ordered enhancers and insulator elements across a subset of NK cluster genes in insects (Cande et al., 2009) lends support to this hypothesis.
Cistron nomenclature is complicated and often confusing for the NK genes. This hinders comparisons beyond species (only see Table 1 for an overview of many of the commonly used names and synonyms for the NK genes). A further problem is that some genes are not easily identified as belonging to a particular cistron family due to low node support values in the phylogenetic trees used to allocate the genes. This has been particularly troublesome for the NK subclass of genes. Ane relevant example in the current context is the difficulty with which the sponge NK cluster genes are identified every bit particular homologs of bilaterian counterparts (Larroux et al., 2007). The A. queenslandica NK cluster is without doubt an NK cluster, but the precise composition of this sponge cluster relative to the bilaterian NK clusters is still open up to some debate due to the lack of robust, clearly resolved molecular phylogenies (Larroux et al., 2007; Fortunato et al., 2014). Thus, information technology is hard to determine the precise composition of the NK cluster in the earliest stages of animal development, before the origin of the bilaterians.
The NK cluster also presents one of the clearest examples yet of the uncertainty that we take most the dynamics and polarity of evolutionary alter in homeobox gene clusters: aboriginal clusters breaking apart vs. dispersed genes coming together (perhaps multiple times independently such that clusters might non be homologous). A recent assay of NK gene locations beyond the densely sampled drosophilids revealed that these genes can come together secondarily by multiple intrachromosomal rearrangements over relatively curt evolutionary periods, i.due east., within genera rather than across phyla, at least for genes that are already linked on the aforementioned chromosome (Chan et al., 2015). In contrast, the presence of NK clusters in sponges, insects and at present hemichordates pushes us to presume that in that location was an ancestral NK cluster formed via the types of tandem duplications and cluster retentivity invoked in hypotheses of the evolution of other homeobox clusters, and and so this ancestral cluster simply disperses (at least to a sure degree) in singled-out lineages. How then can the ii opposing scenarios be reconciled? There is insufficient data and likewise poor an agreement of genome evolutionary dynamics to provide a definitive respond. All the same, ane relevant fact is articulate: not all creature genomes are equal in their evolutionary beliefs, with some genomes evolving and rearranging at much higher rates than others (Irimia et al., 2012). This is about conspicuously exemplified by comparisons of synteny across animals, which reveal that some species showroom loftier (statistically significant) levels of conserved synteny across large evolutionary timescales [e.one thousand., between cnidarians, chordates (Putnam et al., 2007, 2008), some arthropods (Chipman et al., 2014), and lophotrochozoans (Simakov et al., 2013)] whilst other lineages prove loftier rates of rearrangements such that picayune, if any, conserved synteny can be seen fifty-fifty between members of the same phylum [e.g., tunicates (Denoeud et al., 2010) or some insects (Zdobnov and Bork, 2007)]. Consequently, it is articulate that this evolutionary diversity must be taken into account and more homeobox linkage information is required from a taxonomically widespread selection of species in order to distinguish generalities from lineage-specific oddities.
Two farther NK genes are not ordinarily considered as part of the NK cluster, namely Nkx2.1 and Nkx2.2 (for synonyms encounter Table 1). Furthermore, these NK genes tend not to be linked on the same chromosome as the NK cluster genes (Hui et al., 2012), which is taken as a further ancient interchromosomal split of the ancestral Mega-cluster (if this ancestral cluster did actually be; come across above). These genes have now been establish to be components of a "pharyngeal" cistron cluster in some deuterostomes, which has important implications for our understanding of the development of gene clusters more generally.
The Pharyngeal Gene Cluster
The pharyngeal gene cluster was kickoff identified in vertebrates, but has recently been described in other deuterostomes, including hemichordates and an echinoderm (Simakov et al., 2015). This gene cluster gains its name from several of the genes being expressed in the pharyngeal regions of several species in which the cluster is found. It consists of six genes; Nkx2.one, Nkx2.2, Pax1/9, FoxA, mipol1, and slc25A21 (Simakov et al., 2015). Four of the genes are transcription factor-encoding genes, 2 of which comprise homeoboxes (Nkx2.one and Nkx2.2) and one of which is derived from an ancestral homeobox-containing cistron [Pax1/9, which lacks a homeobox whilst other Pax genes take retained some or all of their homeoboxes (Takatori et al., 2008)]. FoxA is the fourth transcription cistron-encoding factor, only is a forkhead domain-encoding gene rather than being from the homeobox superclass. The clustering of these genes seems to be due, at least in part, to the location of regulatory elements of some of the transcription cistron-encoding genes (Pax1/9 and FoxA) within the introns of the two non-transcription gene genes (mipol1 and slc25A21) (Simakov et al., 2015).
One of the distinctive features of this cluster, relative to the clusters discussed above, is that it is non composed of genes that are all related to each other by cistron duplication. Too, Simakov et al. (2015) report that although the cluster can be found in several unlike deuterostomes, it has not still been establish in whatever not-deuterostome and thus is likely to accept evolved specifically in the deuterostome lineage. Information technology will be important to go along investigating whether the pharyngeal cluster is indeed deuterostome-specific, every bit further genome sequences become available, equally discussed further below.
Since orthologs of these pharyngeal cluster genes exercise exist in non-deuterostome animals then it seems this gene cluster constitutes an example of a cluster existence assembled secondarily during evolution. How this and so impacts on our understanding of the homeobox gene clusters described above remains to be seen. Much of the thinking on homeobox clusters has included assumptions that tight physical linkage reflects an ancestral genomic juxtaposition, as described for several of the clusters mentioned in a higher place. This has always seemed reasonable due to the genes being in the aforementioned class or superclass and hence being related via factor duplication. Since the about common course of factor duplication is tandem duplication (Mendivil Ramos and Ferrier, 2012) and so it seems reasonable to suppose that closely neighboring homeobox genes first arose as gene neighbors that have stayed as neighbors in some lineages. This is in contrast to the less parsimonious culling that such genes showtime arose as tandem duplicate neighbors, were and then dispersed effectually the genome during evolution, but secondarily came back together again to exist close neighbors merely in some lineages.
However, perhaps we need to revise our assumptions about such development of genome architecture. The assembly of a functional gene cluster such as the pharyngeal cluster by "pulling genes together" during evolution, rather than tandemly duplicating genes then co-regulating them, provides an important contrast to the homeobox cistron clusters.
Mayhap the pharyngeal cluster tin can be viewed as an extreme version of the co-regulated gene "clusters" such as muscle or firm-keeping genes loosely co-localizing in some animal genomes (Hurst et al., 2004), or groups of genes regulated by the same transcription factors or localizing in the aforementioned nuclear domains of transcriptional activity then coming to lie in the aforementioned regions of genomes post-obit rearrangements during evolution (Janga et al., 2008; Zhang et al., 2012; Farré et al., 2015; Vieux-Rochas et al., 2015). An extension of this evolutionary process might and then have involved the pharyngeal cluster beingness "driven" toward the more farthermost, tighter clustering by further consolidation under overlapping or pan-cluster regulatory mechanisms. Consolidation under long-range, multigenic regulatory mechanisms has been hypothesized for the evolution of vertebrate Hox gene clusters (Duboule, 2007). Also, the evolutionary stabilization of genome neighbors can exist linked to long-range regulatory elements acting on developmental command genes across genomic distances that also happen to harbor neighboring eyewitness genes, as besides seems to be happening for the pharyngeal cluster (Simakov et al., 2015). However, how "difficult" or "piece of cake" it is for such arrangements to evolve, and tight clusters of functionally related genes be assembled secondarily, still needs to be examined more than widely across the animals. Likewise, if such a "secondary" evolutionary process is to exist invoked for homeobox clusters such as the Hox, ParaHox, NK, and so on, then it will be necessary to found the boosted likelihood of tandemly duplicated genes dispersing prior to so meeting again secondarily in a process comparable to the associates of the pharyngeal cluster.
At that place is an additional gene that should mayhap also be considered in the context of the pharyngeal cluster: Msxlx. Although Simakov et al. (2015) exercise non formally include this homeobox gene in the pharyngeal cluster, they do show that it is present in the clusters of hemichordates and the echinoderm Acanthaster planci. Msxlx is also clustered with Nkx2.2 in the protostome Lottia gigantea (Simakov et al., 2015). This is intriguing, and indicates that it is definitely necessary to await more than closely across a wider range of species before we conclude that the pharyngeal cluster definitely does represent a deuterostome-specific entity (rather than simply a cluster that has dispersed in the limited range of non-deuterostomes examined to engagement). Examination of the expression of Msxlx in a range of species is as well required. The expression has been studied in the invertebrate chordate amphioxus (Branchiostoma floridae; Butts et al., 2010). Butts et al. (2010) focused on Msxlx because it is one of a pocket-sized handful of homeobox genes that have been lost during the development of the Olfactores (i.due east., the urochordates plus vertebrates). This accounts for why information technology is not found in the pharyngeal clusters of vertebrates, but, more than importantly, the expression in amphioxus exhibits an intriguing association with the pharyngeal region (as do the other "lost" homeobox genes investigated by Butts et al., 2010). Amphioxus Msxlx is expressed in the region of the anterior endoderm that constitutes Hatschek's left diverticulum, and develops into the pre-oral pit past fusing with the ectoderm. This is idea to exist homologous to the vertebrate adenohypophysis. The genes of the pharyngeal cluster, including Msxlx, are thus an interesting group of genes to investigate farther for two main reasons. Firstly, the evolution of their genomic system is intriguing, for the potential for improving our understanding of gene cluster evolution. Secondly, the evolution of their expression is interesting in the context of understanding the evolution of the pharyngeal region.
Conclusion
The instances of homeobox factor clustering discussed above are focused on those that are already described in, or tin exist gleaned from, the literature. There are likely to be additional instances of homeobox clustering to exist plant in the ever-increasing number of whole genome sequences that are becoming bachelor, which will enable farther refinement of the clusters described here every bit well equally possibly providing new examples of clusters that had aboriginal origins but accept thus far been disregarded. It is valuable to proceed to search for such clusters as they provide important insights into evolutionary transitions, both in terms of beast development likewise as genome organization. Such links between genome organization, as represented by cluster arrangement, and the evolution of animal development have been the focus of much attention for the renowned Hox genes, almost ever since their discovery in the 1980s. The further homeobox clusters discussed hither provide a whole new suite of opportunities to expand the study systems available to us for such evolutionary developmental genomics research. Such research is too vital if nosotros are to empathize the evolutionary dynamics of animal genomes and distinguish primary from secondary clustering.
Author Contributions
DF conceived and wrote the manuscript.
Funding
Work in the writer'due south lab is funded by BBSRC DTP studentships and the Schoolhouse of Biology, University of St. Andrews.
Conflict of Involvement Statement
The writer declares that the research was conducted in the absence of any commercial or fiscal relationships that could exist construed every bit a potential disharmonize of interest.
Acknowledgments
The writer would like to thank past and nowadays members of the lab for discussions likewise as colleagues in the community. The referees as well provided a number of helpful comments that improved the manuscript.
References
Butts, T., Holland, P. W. H., and Ferrier, D. E. K. (2010). Ancient homeobox factor loss and the evolution of chordate brain and pharynx development: deductions from amphioxus gene expression. Proc. Biol. Sci. 277, 3381–3389. doi: 10.1098/rspb.2010.0647
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cande, J. D., Chopra, V. S., and Levine, 1000. (2009). Evolving enhancer-promoter interactions within the tinman circuitous of the flour protrude, Tribolium castaneum. Evolution 136, 3153–3160. doi: 10.1242/dev.038034
PubMed Abstruse | CrossRef Full Text | Google Scholar
Castro, F. L., and Kingdom of the netherlands, P. W. H. (2003). Chromosomal mapping of ANTP class homeobox genes in amphioxus: piecing together ancestral genomes. Evol. Dev. 5, 459–465. doi: ten.1046/j.1525-142X.2003.03052.x
PubMed Abstract | CrossRef Total Text | Google Scholar
Chan, C., Jayasekera, S., Kao, B., Pàramo, M., von Grotthuss, G., and Ranz, J. Chiliad. (2015). Remodelling of a homeobox factor cluster by multiple independent gene reunions in Drosophila. Nat. Commun. six:6509. doi: 10.1038/ncomms7509
PubMed Abstruse | CrossRef Full Text | Google Scholar
Chipman, A. D., Ferrier, D. E. K., Brena, C., Qu, J., Hughes, D. Due south. T., Schröder, R., et al. (2014). The first myriapod genome sequence reveals conservative arthropod factor content and genome organisation in the centipede Strigamia maritima. PLoS Biol. 12:e1002005. doi: 10.1371/journal.pbio.1002005
PubMed Abstract | CrossRef Total Text | Google Scholar
Degnan, B. M., Vervoort, M., Larroux, C., and Richards, G. S. (2009). Early evolution of metazoan transcription factors. Curr. Opin. Genet. Dev. xix, 591–599. doi: x.1016/j.gde.2009.09.008
PubMed Abstruse | CrossRef Full Text | Google Scholar
de Mendoza, A., Sebé-Pedrósa, A., Sestak, M. South., Matejcic, Chiliad., Torruella, G., Domazet-Loso, T., et al. (2013). Transcription factor evolution in eukaryotes and the assembly of the regulatory toolkit in multicellular lineages. Proc. Natl. Acad. Sci. UsA. 110, E4858–E4866. doi: 10.1073/pnas.1311818110
PubMed Abstract | CrossRef Total Text | Google Scholar
Denoeud, F., Henriet, S., Mungpakdee, S., Aury, J-M., Da Silva, C., Brinkmann, H., et al. (2010). Plasticity of animal genome compages unmasked by rapid evolution of a pelagic tunicate. Science 330, 1381–1385. doi: 10.1126/science.1194167
PubMed Abstract | CrossRef Full Text | Google Scholar
Derelle, R., Lopez, P., Le Guyader, H., and Manuel, Grand. (2007). Homeodomain proteins belong to the ancestral molecular toolkit of eukaryotes. Evol. Dev. nine, 212–219. doi: 10.1111/j.1525-142X.2007.00153.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Duboule, D. (1994). Guidebook to the Homeobox Genes. Oxford: Oxford University Printing.
Google Scholar
Farré, Thou., Robinson, T. J., and Ruiz-Herrera, A. (2015). An Integrative Breakage Model of genome architecture, reshuffling and evolution: the Integrative Breakage Model of genome evolution, a novel multidisciplinary hypothesis for the study of genome plasticity. BioEssay 37, 479–488. doi: 10.1002/bies.201400174
PubMed Abstract | CrossRef Total Text | Google Scholar
Ferrier, D. E. Grand. (2008). "When is a Hox gene not a Hox gene? The importance of gene nomenclature," in Evolving Pathways: Central Themes in Evolutionary Developmental Biology, eds A. Minelli and G. Fusco (Cambridge: Cambridge University Printing), 175–193.
Ferrier, D. East. K. (2010). "Evolution of Hox complexes," in Hox Genes: Studies from the 20 th to the 21 st Century, ed J. S. Deutsch (Austin, TX; New York, NY: Landes Bioscience and Springer Scientific discipline and Business Media), 91–100.
Google Scholar
Ferrier, D. E. K. (2012). Evolution of the Hox Cistron Cluster. Chichester: eLS. John Wiley & Sons, Ltd.
Google Scholar
Ferrier, D. E. One thousand., Brooke, N. M., Panopoulou, G., and Holland, P. Westward. H. (2001). The Mnx homeobox gene class defined by HB9, MNR2 and amphioxus AmphiMnx. Dev. Genes Evol. 211, 103–107. doi: ten.1007/s004270000124
PubMed Abstract | CrossRef Full Text | Google Scholar
Ferrier, D. E. Thousand., and Holland, P. W. H. (2002). Ciona intestinalis ParaHox genes: development of Hox/ParaHox cluster integrity, developmental mode and temporal colinearity. Mol. Phylogenet. Evol. 24, 412–417. doi: ten.1016/S1055-7903(02)00204-X
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ferrier, D. Due east. K., and Minguillón, C. (2003). Development of the Hox/ParaHox factor clusters. Int. J. Dev. Biol. 47, 605–611.
PubMed Abstract | Google Scholar
Fortunato, Southward. A., Adamski, Thousand., Mendivil Ramos, O., Leininger, S., Liu, J., Ferrier, D. E. K., et al. (2014). Calcisponges accept a ParaHox cistron and dynamic expression of dispersed NK homeobox genes. Nature 514, 620–623. doi: 10.1038/nature13881
PubMed Abstruse | CrossRef Full Text | Google Scholar
Friedrich, M. (2015). Evo-devo gene toolkit update: at least 7 Pax transcription factor subfamilies in the last mutual ancestor of bilaterian animals. Evol. Dev. 17, 255–257. doi: 10.1111/ede.12137
PubMed Abstract | CrossRef Full Text | Google Scholar
Gómez-Marín, C., Tena, J. J., Acemel, R. D., López-Mayorga, Thou., Naranjo, S., de la Calle-Mustienes, East., et al. (2015). Evolutionary comparison reveals that diverging CTCF sites are signatures of bequeathed topological associating domains borders. Proc. Natl. Acad. Sci. U.s.a.A. 112, 7542–7547. doi: x.1073/pnas.1505463112
PubMed Abstruse | CrossRef Total Text | Google Scholar
Hui, J. H. L., McDougall, C., Monteiro, A. S., Holland, P. W. H., Arendt, D., Balavoine, G., et al. (2012). Extensive chordate and annelid macrosynteny reveals bequeathed homeobox gene organization. Mol. Biol. Evol. 29, 157–165. doi: 10.1093/molbev/msr175
PubMed Abstruse | CrossRef Full Text | Google Scholar
Irimia, Chiliad., Maeso, I., and Garcia-Fernàndez, J. (2008). Convergent development of clustering of Iroquois homeobox genes across metazoans. Mol. Biol. Evol. 25, 1521–1525. doi: 10.1093/molbev/msn109
PubMed Abstruse | CrossRef Total Text | Google Scholar
Irimia, M., Tena, J. J., Alexis, Chiliad. South., Fernandez-Miñan, A., Maeso, I., Bogdanovic, O., et al. (2012). Extensive conservation of ancient microsynteny across metazoans due to cis-regulatory constraints. Genome Res. 22, 2356–2367. doi: 10.1101/gr.139725.112
PubMed Abstract | CrossRef Full Text | Google Scholar
Jagla, K., Bellard, M., and Frasch, M. (2001). A cluster of Drosophila homeobox genes involved in mesoderm differentiation programs. BioEssays 23, 125–133. doi: 10.1002/1521-1878(200102)23:two<125::AID-BIES1019>3.0.CO;2-C
PubMed Abstract | CrossRef Total Text | Google Scholar
Janga, S. C., Collado-Vides, J., and Babu, M. Thousand. (2008). Transcriptional regulation constrains the organization of genes on eukaryotic chromosomes. Proc. Natl. Acad. Sci. UsA. 105, 15761–15766. doi: 10.1073/pnas.0806317105
PubMed Abstract | CrossRef Full Text | Google Scholar
Kamm, K., and Schierwater, B. (2007). Ancient linkage of a POU form six and an anterior Hox-similar gene in Cnidaria: implications for the evolution of homeobox genes. J. Exp. Zool. B Mol. Dev. Evol. 308, 777–784. doi: 10.1002/jez.b.21196
PubMed Abstract | CrossRef Full Text | Google Scholar
Kenchappa, C. Southward., Heidarsson, P. O., Kragelund, B. B., Garrett, R. A., and Poulsen, F. G. (2013). Solution backdrop of the archaeal CRISPR DNA echo-bounden homeodomain protein Cbp2. Nucl. Acid Res. 41, 3424–3435. doi: 10.1093/nar/gks1465
PubMed Abstract | CrossRef Full Text | Google Scholar
Kikuta, H., Laplante, M., Navratilova, P., Komisarczuk, A. Z., Engström, P. G., Fredman, D., et al. (2007). Genomic regulatory blocks encompass multiple neighboring genes and maintain conserved synteny in vertebrates. Genome Res. 17, 545–555. doi: ten.1101/gr.6086307
PubMed Abstract | CrossRef Full Text | Google Scholar
King, Northward., Westbrook, Grand. J., Young, S. Fifty., Kuo, A., Abedin, Thousand., Chapman, J., et al. (2008). The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451, 783–788. doi: 10.1038/nature06617
PubMed Abstract | CrossRef Full Text | Google Scholar
Larroux, C., Fahey, B., Degnan, S. M., Adamski, One thousand., Rokhsar, D. Southward., and Degnan, B. M. (2007). The NK homeobox gene cluster predates the origin of hox genes. Curr. Biol. 17, 706–710. doi: 10.1016/j.cub.2007.03.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Larroux, C., Luke, Yard. N., Koopman, P., Rokhsar, D. S., Shimeld, S. M., and Degnan, B. Yard. (2008). Genesis and expansion of metazoan transcription factor gene classes. Mol. Biol. Evol. 25, 980–996. doi: ten.1093/molbev/msn047
PubMed Abstract | CrossRef Full Text | Google Scholar
Luke, 1000. N., Castro, L. F., McLay, K., Bird, C., Coulson, A., and Holland, P. Due west. H. (2003). Dispersal of NK homeobox cistron clusters in amphioxus and humans. Proc. Natl. Acad. Sci. U.Due south.A. 100, 5292–5295. doi: 10.1073/pnas.0836141100
PubMed Abstract | CrossRef Full Text | Google Scholar
Maeso, I., Irimia, M., Tena, J. J., González-Pérez, E., Tran, D., Ravis, Five., et al. (2012). An ancient genomic regulatory cake conserved across bilaterians and its dismantling in tetrapods by retrogene replacement. Genome Res. 22, 642–655. doi: 10.1101/gr.132233.111
PubMed Abstract | CrossRef Total Text | Google Scholar
Mazza, M. E., Pang, Thou., Reitzel, A. One thousand., Martindale, M. Q., and Finnerty, J. R. (2010). A conserved cluster of iii PRD-class homeobox genes (homeobrain, rx and orthopedia) in the Cnidaria and Protostomia. EvoDevo 1:iii. doi: ten.1186/2041-9139-i-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Mendivil Ramos, O., and Ferrier, D. E. K. (2012). Mechanisms of gene duplication and translocation and progress towards understanding their relative contributions to fauna genome evolution. Int. J. Evol. Biol. 2102:846421. doi: 10.1155/2012/846421
CrossRef Full Text | Google Scholar
Mukherjee, 1000., Brocchieri, L., and Bürglin, T. R. (2009). A comprehensive classification and evolutionary analysis of establish homeobox genes. Mol. Biol. Evol. 26, 2775–2794. doi: ten.1093/molbev/msp201
PubMed Abstract | CrossRef Full Text | Google Scholar
Mukherjee, M., and Bürglin, T. R. (2007). Comprehensive analysis of beast TALE homeobox genes: new conserved motifs and cases of accelerated evolution. J. Mol. Evol. 65, 137–153. doi: 10.1007/s00239-006-0023-0
PubMed Abstruse | CrossRef Full Text | Google Scholar
Narendra, V., Rocha, P. P., An, D., Raviram, R., Skok, J. A., Mazzoni, E. O., et al. (2015). CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science 347, 1017–1021. doi: 10.1126/science.1262088
PubMed Abstract | CrossRef Full Text | Google Scholar
Putnam, N. H., Butts, T., Ferrier, D. East. K., Furlong, R. F., Hellsten, U., Kawashima, T., et al. (2008). The amphioxus genome and the development of the chordate karyotype. Nature 453, 1064–1071. doi: ten.1038/nature06967
PubMed Abstract | CrossRef Total Text | Google Scholar
Putnam, Due north. H., Srivastava, M., Hellsten, U., Dirks, B., Chapman, J., Salamov, A., et al. (2007). Bounding main anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94. doi: ten.1126/science.1139158
PubMed Abstract | CrossRef Full Text | Google Scholar
Ryan, J. F., Burton, P. M., Mazza, M. E., Kwong, Thousand. K., Mullikin, J. C., and Finnerty, J. R. (2006). The cnidarian-bilaterian ancestor possessed at least 56 homeoboxes: evidence from the startlet body of water anemone, Nematostella vectensis. Genome Biol. 7:R64. doi: x.1186/gb-2006-seven-7-r64
PubMed Abstract | CrossRef Total Text | Google Scholar
Schierwater, B., Kamm, K., Srivastava, 1000., Rokhsar, D., Rosengarten, R. D., and Dellaporta, S. Fifty. (2008). The early on ANTP cistron repertoire: insights from the placozoan genome. PLoS Ane 3:e2457. doi: 10.1371/periodical.pone.0002457
PubMed Abstract | CrossRef Full Text | Google Scholar
Schmidt-Ott, U., Rafiqi, A. Thou., and Lemke, S. (2010). "Hox3/zen and the evolution of extraembryonic epithelia in insects," in Hox Genes: Studies from the 20th to the 21st Century, ed J. S. Deutsch (Austin, TX; New York, NY: Landes Bioscience and Springer Scientific discipline+Business Media).
Google Scholar
Sebé-Pedrós, A., de Mendoza, A., Lang, B. F., Degnan, B. M., and Ruiz-Trillo, I. (2011). Unexpected repertoire of metazoan transcription factors in the unicellular holozoan Capsaspora owczarzaki. Mol. Biol. Evol. 28, 1241–1254. doi: ten.1093/molbev/msq309
PubMed Abstract | CrossRef Full Text | Google Scholar
Simakov, O., Kawashima, T., Marlétaz, F., Jenkins, J., Koyanagi, R., Mitros, T., et al. (2015). Hemichordate genomes and deuterostome origins. Nature 527, 459–465. doi: 10.1038/nature16150
PubMed Abstract | CrossRef Full Text | Google Scholar
Simakov, O., Marlétaz, F., Cho, Due south. J., Edsinger-Gonzales, East., Havlak, P., Hellsten, U., et al. (2013). Insights into bilaterian evolution from three spiralian genomes. Nature 493, 526–531. doi: 10.1038/nature11696
PubMed Abstract | CrossRef Full Text
Srivastava, M., Larroux, C., Lu, D. R., Mohanty, K., Chapman, J., Degnan, B. G., et al. (2010). Early on evolution of the LIM homeobox factor family. BMC Biol. 8:4. doi: 10.1186/1741-7007-8-iv
PubMed Abstract | CrossRef Full Text | Google Scholar
Suga, H., Chen, Z., de Mendoza, A., Sebé-Pedrós, A., Brown, Yard. W., Kramer, E., et al. (2013). The Capsaspora genome reveals a complex unicellular prehistory of animals. Nat. Commun. 4, 2325. doi: x.1038/ncomms3325
PubMed Abstract | CrossRef Full Text | Google Scholar
Takatori, N., Butts, T., Candiani, S., Pestarino, M., Ferrier, D. E. Thou., Saiga, H., et al. (2008). Comprehensive survey and classification of homeobox genes in the genome of amphioxus, Branchiostoma floridae. Dev. Genes Evol. 218, 579–590. doi: 10.1007/s00427-008-0245-ix
PubMed Abstract | CrossRef Total Text | Google Scholar
Vieux-Rochas, M., Fabre, P. J., Leleu, M., Dubole, D., and Noordermeer, D. (2015). Clustering of mammalian Hox genes with other H3K27me3 targets within an agile nuclear domain. Proc. Natl. Acad. Sci. U.Due south.A. 112, 4672–4677. doi: 10.1073/pnas.1504783112
PubMed Abstract | CrossRef Full Text | Google Scholar
Wotton, M. R., Weierud, F. Yard., Juárez-Morales, J. Fifty., Alvares, L. East., Dietrich, D., and Lewis, K. East. (2009). Conservation of cistron linkage in dispersed vertebrate NK homeobox clusters. Dev. Genes Evol. 219, 481–496. doi: 10.1007/s00427-009-0311-y
PubMed Abstract | CrossRef Total Text | Google Scholar
Zhang, Y., McCord, R. P., Ho, Y-J., Lajoie, B. R., Hildebrand, D. G., Simon, A. C., et al. (2012). Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 148, 908–921. doi: 10.1016/j.cell.2012.02.002
PubMed Abstract | CrossRef Full Text | Google Scholar
Zhong, Y-F., and Holland, P. Westward. H. (2011). HomeoDB2: functional expansion of a comparative homeobox gene database for evolutionary developmental biological science. Evol. Dev. 13, 567–568. doi: 10.1111/j.1525-142X.2011.00513.x
PubMed Abstruse | CrossRef Total Text | Google Scholar
jeffersonwhingent.blogspot.com
Source: https://www.frontiersin.org/articles/183245
0 Response to "Hox Is a Gene Family That Determines Skin or Cuticle Coloration in a Wide Range of Animals."
Postar um comentário